 The battery is then filled with electrolyte - or battery acid -- a mixture of sulfuric acid and water, and the cover is attached. The battery is checked for leaks. The battery is then filled with electrolyte - or battery acid -- a mixture of sulfuric acid and water, and the cover is attached. The battery is checked for leaks.
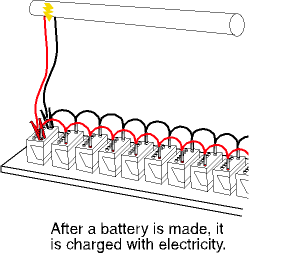
The final step is charging, or finishing. During this step, the battery terminals are connected to a source of electricity and the battery is charged for many hours. When the battery is fully charged, it moves to another line where the case is cleaned, if necessary, and the labels are attached.
A battery stores electricity for future use. It develops voltage from the chemical reaction produced when two unlike materials, such as the positive and negative plates, are immersed in the electrolyte, a solution of sulfuric acid and water. In a typical lead-acid battery, the voltage is approximately 2 volts per cell, for a total of 12 volts. Electricity flows from the battery as soon as there is a circuit between the positive and negative terminals. This happens when any load that needs electricity, such as the radio, is connected to the battery.
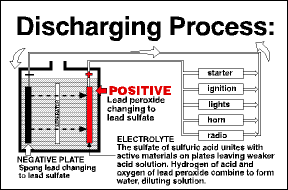 Most people don’t realize that a lead-acid battery operates in a constant process of charge and discharge. When a battery is connected to a load that needs electricity, such as the engine starter, current flows from the battery. The battery begins to be discharged. Most people don’t realize that a lead-acid battery operates in a constant process of charge and discharge. When a battery is connected to a load that needs electricity, such as the engine starter, current flows from the battery. The battery begins to be discharged.
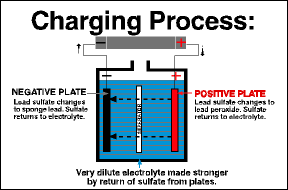
In the reverse process, a battery becomes charged when current flows back into it, restoring the chemical difference between the plates. This happens when you’re driving without any accessories and the alternator puts current back into the battery.
 As a battery discharges, the lead plates become more chemically alike, the acid becomes weaker, and the voltage drops. Eventually the battery is so discharged that it can no longer deliver electricity at a useful voltage As a battery discharges, the lead plates become more chemically alike, the acid becomes weaker, and the voltage drops. Eventually the battery is so discharged that it can no longer deliver electricity at a useful voltage
You can recharge a discharged battery by feeding electrical current back into the battery. A full charge restores the chemical difference between the plates and leaves the battery ready to deliver its full power.
This unique process of discharge and charge in the lead-acid battery means that energy can be discharged and restored over and over again. This is what’s known as the cycling ability in a battery.
Each cell in a battery undergoes the same two chemical processes to create voltage within the cell. In its simplest form, two sheets of lead are housed in the acid resistant tank filled with diluted sulfuric acid (electrolyte). In this state nothing happens, the plates just sit there. However, if you add a current, such as from a battery charger, chemical reactions start to occur. The lead plate attached to the positive (+) side of the battery charger starts to turn brown as the lead surface is turned into lead peroxide. Simultaneously, the lead plate attached to the negative (-) side of the battery charger forms an oxide film that is plain metallic lead. When this chemical reaction has occurred the cell is now charged. If you use a multimeter to measure the voltage you will get a reading of approximately 2 volts.
|